ACADEMIA
Cells in blood vessel found to cling more tightly in regions of rapid flow
Clogging of pipes leading to the heart is the planet's number one killer. Surgeons can act as medical plumbers to repair some blockages, but we don't fully understand how this living organ deteriorates or repairs itself over time.
Researchers at the University of Washington have studied vessel walls and found the cells pull more tightly together, reducing vascular leakage, in areas of fast-flowing blood. The finding could influence how doctors design drugs to treat high cholesterol, or how cardiac surgeons plan their procedures.
Their paper will be published in an upcoming issue of the American Journal of Physiology - Heart and Circulatory Physiology.
"Our results indicate that these cells can sense the kind of flow that they're in, and structurally change how they hold themselves together," said lead author Nathan Sniadecki, a UW assistant professor of mechanical engineering. "This highlights the role that cellular forces play in the progression of cardiovascular disease."
It's known that the arteries carrying blood are leakier in areas of slow flow, promoting cholesterol buildup in those areas. But medical researchers believed this leakage was mostly biochemical – that cells would sense the slower flow and modify how proteins and enzymes function inside the cell to allow for more exchange.
The new results show that, like a group of schoolchildren huddling closer in a gust of wind, the cells also pull more tightly together when the blood is flowing past.
"The mechanical tugging force leads to a biochemical change that allows more and more proteins at the membrane to glue together," Sniadecki said. "We're still trying to understand what's happening here, and how mechanical tugging leads more proteins to localize and glue at the interface."
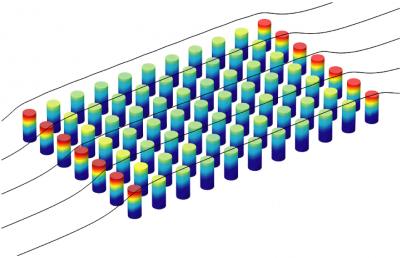
Sniadecki's group looks at the biomechanics of individual cells. For this experiment, they grew a patch of human endothelial cells, the thin layer of cells that line the inner walls of arteries and veins and act as a sort of nonstick coating for the vessels' walls. They grew the patch on an area about the width of a human hair, manufactured with 25 by 25 tiny flexible silicon posts.
The researchers then looked at how much the cells bend the posts under different flow conditions in order to calculate how strongly the cells were tugging on their neighbors. When the flow was fast, the force between the cells increased, while the gaps between cells shrank.
Knowing how cells respond to blood flow could help find new drugs to promote this tugging between cells. Better understanding of the interaction between blood flow and heart health could also guide surgeries.
"People could do simulations so a surgeon goes, 'Ah, I should cut here versus over here, because that reconstruction will be a smoother vessel and will lead to fewer complications down the line, or as I put this stent in, put it here and make it more aerodynamic in design,'" Sniadecki said.