THE LATEST
UVA researchers claim to crack the autism code
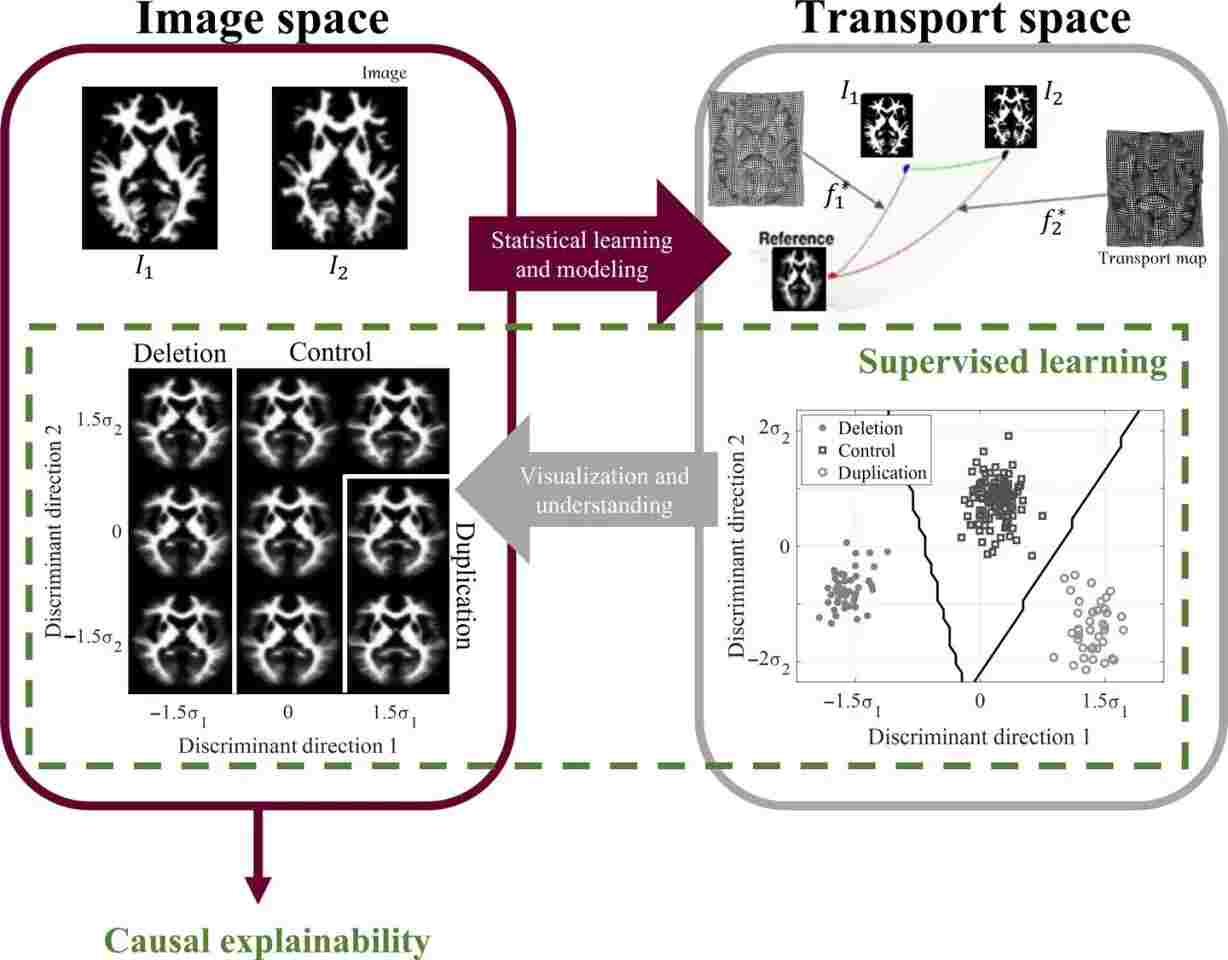
In a breakthrough that could revolutionize the diagnosis and treatment of autism, researchers from the University of Virginia claim to have decoded the neurodivergent brain. Using mathematical modeling techniques, their system can reportedly identify genetic markers of autism in brain images with remarkable accuracy. While this advancement holds potential benefits, it also raises challenging ethical concerns.
The multi-university research team, led by engineering professor Gustavo K. Rohde, has achieved accuracy rates between 89 to 95% in identifying autism-related genetic markers solely from brain scans. They argue that their approach, which focuses on genetic information rather than behavioral cues, could lead to earlier interventions and personalized medicine.
Traditional autism diagnoses heavily rely on behavioral observations, leading to delays in identification and intervention. If this new system proves effective, doctors could potentially bypass the need for behavioral assessments, allowing for quicker and more accurate diagnoses. Such progress may offer hope for individuals and families affected by autism.
However, relying solely on brain imaging and genetic markers for diagnosis and treatment raises concerns that cannot be overlooked. Critics caution that reducing autism to a solely genetic phenomenon oversimplifies the complex interplay between genetics, environment, and behavioral factors.
Moreover, the use of machine learning and mathematical modeling techniques, such as transport-based morphometry (TBM), brings its own set of challenges. While TBM claims to extract mass transport information from medical images, there are concerns about the reliability and validity of these mathematical models. Skeptics argue that this approach may overlook or misinterpret vital data that could potentially lead to misdiagnoses or inadequate treatments.
Additionally, the research team utilized data from the Simons Variation in Individuals Project, a specific group with autism-linked genetic variations. This lack of diversity in study participants raises concerns about the generalizability of the findings and the potential exclusion of individuals with different genetic profiles or diverse backgrounds.
The researchers acknowledge that understanding the biological basis of autism is a complex task but assert that their method represents a crucial step in unraveling the gene-brain-behavior relationship. However, it is crucial to approach these claims with cautious investigation and recognize the limitations of this approach.
The potential impact of this research is significant, as it aims to transform the landscape of autism diagnosis and treatment. Yet, the medical community must navigate the ethical implications of relying solely on brain imaging and genetic markers, ensuring that personalized medicine does not overlook the unique needs and experiences of neurodivergent individuals.
As we embrace the promise of this breakthrough, it is imperative that we also engage in a critical dialogue about the broader implications and potential risks associated with such advancements. Only through careful consideration and holistic approaches can we move towards a future that supports and empowers all individuals, regardless of their neurological differences.

