ENGINEERING
Pitt researchers create model to predict the need for surgery in Abdominal Aortic Aneurysms
An abdominal aortic aneurysm (AAA) can be a ticking time bomb if undiscovered in time. However, researchers at the University of Pittsburgh are developing a new model to better predict at-risk patients. And the tools they are using apply mechanical testing to the human body - which is itself a complex machine.
An AAA occurs when the aorta weakens and begins to irreversibly dilate, like a slowly inflating balloon. If left untreated, the risk of rupture increases and has a 90 percent rate of mortality, making AAA the 15th leading cause of death in the United States with more than 15,000 deaths reported annually.
Once diagnosed, clinicians must determine whether the aorta requires surgery, using the AAA diameter to decide if an aneurysm is clinically relevant. A diameter 5.5 centimeters or larger typically calls for surgical intervention, barring other contraindications, but this one-size-fits-all approach misses nearly 25 percent of patients who experience a rupture at a smaller size. 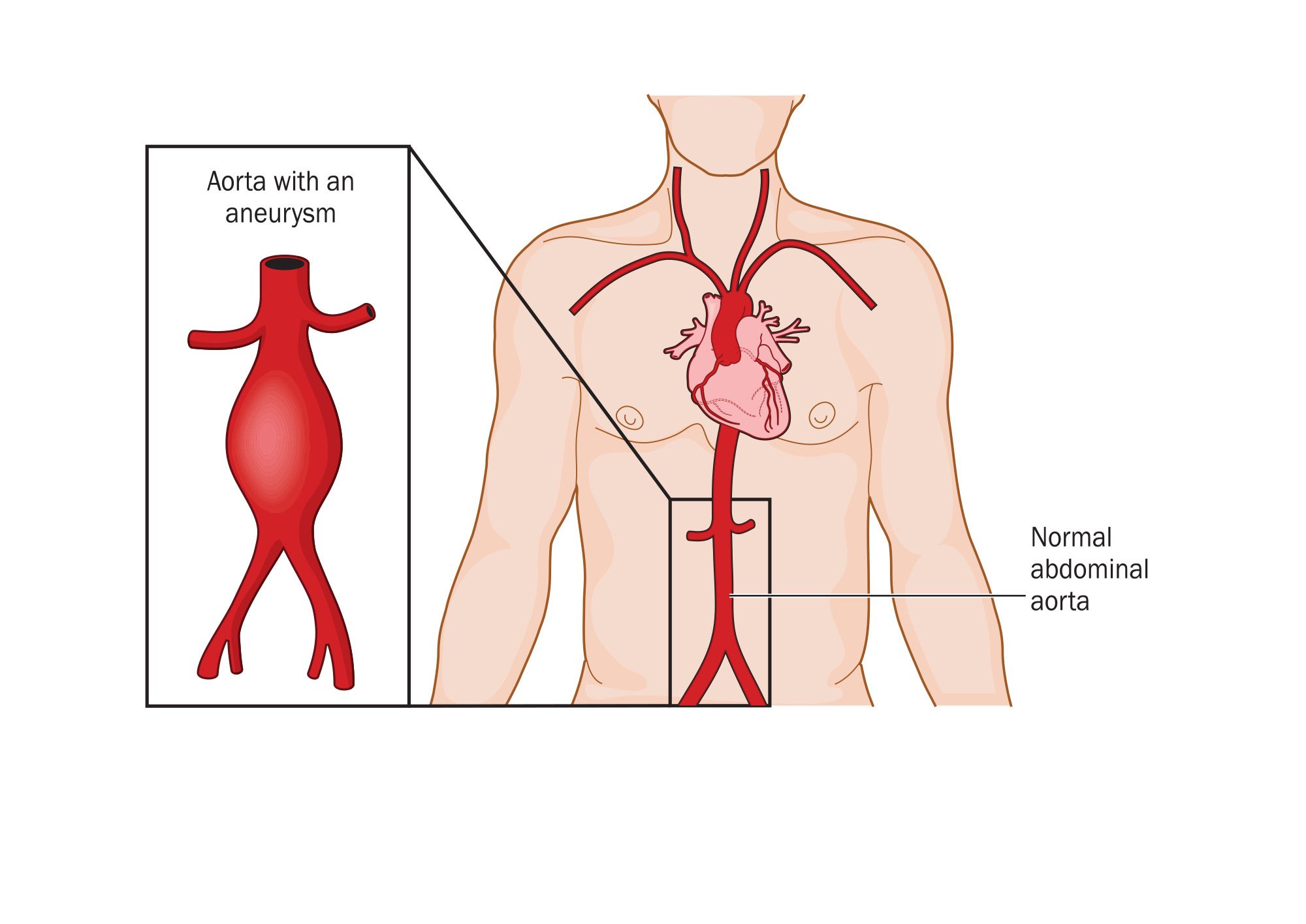 {module INSIDE STORY}
{module INSIDE STORY}
Pitt bioengineer David A. Vorp received an award from the National Institutes of Health to track the natural evolution of small AAA and develop a predictive model to improve patient prognosis. His Vascular Bioengineering Lab at the university's Swanson School of Engineering is focused on finding novel diagnoses and treatments for these silent killers.
"It's a ticking time bomb," explained Timothy Chung, a post-doctoral associate in Vorp's lab. "Once you diagnose an abdominal aortic aneurysm, you don't know when or if it's going to rupture.
"Imagine you're blowing up a balloon, and it pops. This event involves the mechanics and forces that are interacting with the wall of the balloon," continued Chung, who will help lead the project. "We're interested in the biomechanics of why elevated pressure or a weakening of the aneurysm wall might lead to rupture or accelerated growth."
The research team hopes that CT scans and other data from a rare, longitudinal clinical trial ("Non-Invasive Treatment of Abdominal Aortic Aneurysm Clinical Trial") will help them identify the risks of elevated growth rate or eventual rupture.
Vorp's lab group will create 3D geometric reconstructions and perform biomechanical simulations on patient datasets at each imaging scan interval (every six months) to learn how small AAA progresses over time. They will then use the scans and unique software tools from their lab to perform shape analyses that will determine which geometries may lead to poor patient outcomes.
"Currently, clinicians are simply applying a one-dimensional shape analysis, using the diameter as a threshold for clinical intervention," said Chung. "The tools developed in the Vascular Bioengineering Lab can help us extract more than one-dimensional measurements. They allow us to create two- and three-dimensional shape indices derived from image-based surface reconstructions, allowing for a more robust analysis."
The team will then feed data from the shape analysis and biomechanical simulations to train a machine-learning algorithm to classify different types of aneurysm outcomes. This will be used to develop a predictive model that can help guide clinicians and determine the need for surgical intervention.
"Early in my career, the advent of finite element analysis - a computational method to predict mechanical wall stress distribution in complex shapes both biological and human-made - provided a game-changing tool to better understand the role of biomechanics in AAA disease," said Vorp, Associate Dean for Research and John A. Swanson Professor of Bioengineering. "Now, machine learning technologies can not only help us better understand the combination of factors that lead toward rupture or clinical intervention but also package that knowledge into a true, personalized health tool for those afflicted with this potentially lethal condition."

