BIG DATA
Surrey's Xavier performs simulations to produce discoveries for sustainable hydrogen production
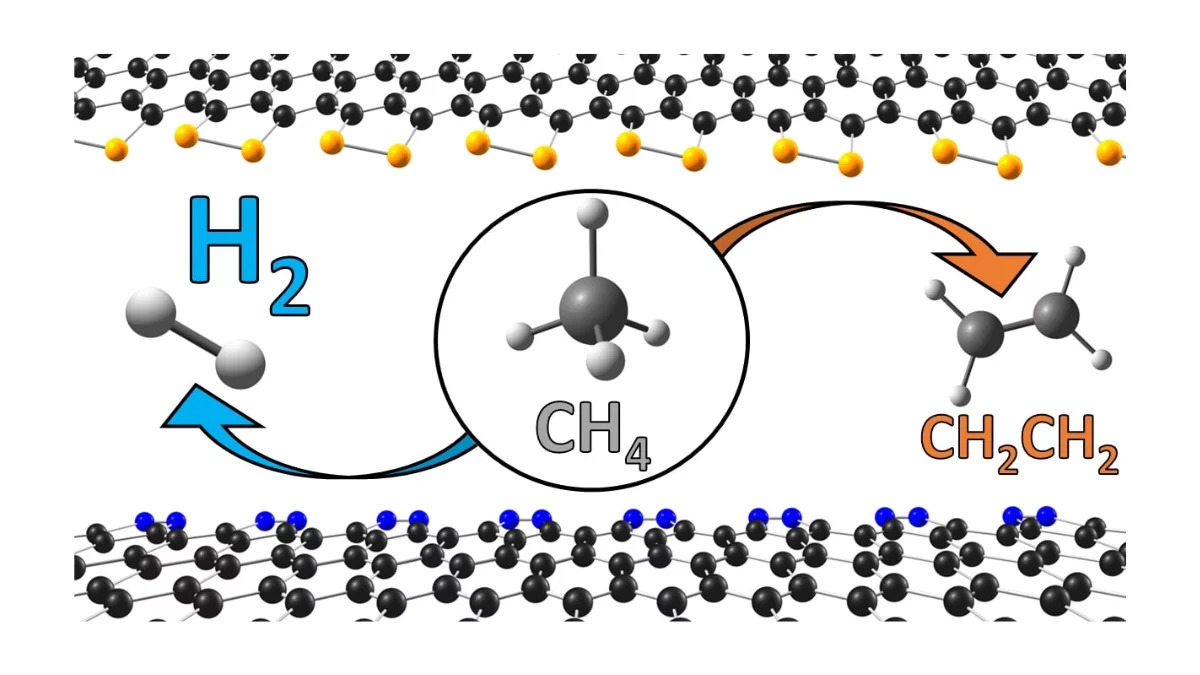
Hydrogen fuel could be a more viable alternative to traditional fossil fuels according to the University of Surrey researchers in Guildford, Surrey, England who have found that a type of metal-free catalysts could contribute to the development of cost-effective and sustainable hydrogen production technologies.
The study has shown promising results for the use of edge-decorated nano carbons as metal-free catalysts for the direct conversion of methane, which is also a powerful greenhouse gas, into hydrogen. Among the nano carbons investigated, nitrogen-doped nano carbons presented the highest level of performance for hydrogen production at high temperatures.
Crucially, the researchers also found that the nitrogen-doped and phosphorous-doped nano carbons had strong resistance to carbon poisoning, which is a common issue with catalysts in this process.
Dr. Neubi Xavier Jr, the Research Fellow who performed the material science simulations, said: "Our results suggest that using edge-decorated nano carbons as catalysts could be a game-changer for the hydrogen industry, offering a cost-effective and sustainable alternative to traditional metal catalysts. At the same time, this process gets rid of methane, which is a fossil fuel involved in global warming."
Hydrogen fuel is a clean and renewable energy source that has the potential to reduce carbon emissions and decrease our dependence on fossil fuels. When used as a fuel, hydrogen can power vehicles, generate electricity, and heat buildings. The only by-product of hydrogen fuel is water vapor, making it an environmentally friendly alternative to traditional fossil fuels.
However, the production of hydrogen fuel is currently reliant on fossil fuels, which creates carbon emissions in the process, and metal catalysts, which mining and manufacturing are energy intensive and can negatively affect the environment. Therefore, the development of sustainable hydrogen production methods and catalytic materials is crucial to realizing the full potential of hydrogen fuel as a clean energy source.
The research was conducted by a team led by Dr. Marco Sacchi from the University of Surrey, an expert in the field of sustainable energy and computational chemistry, who combined quantum chemistry, thermodynamics, and chemical kinetics to determine the most efficient edge decoration for hydrogen production.
Dr. Sacchi said: "One of the biggest challenges with catalysts for hydrogen production is that they can get poisoned by carbon. But our study found that nitrogen and phosphorous-doped nano carbons are pretty resistant to this problem. This is a huge step forward for sustainable hydrogen production."

