INDUSTRY
NC State supercomputer simulations design more efficient amine chemical scrubbers
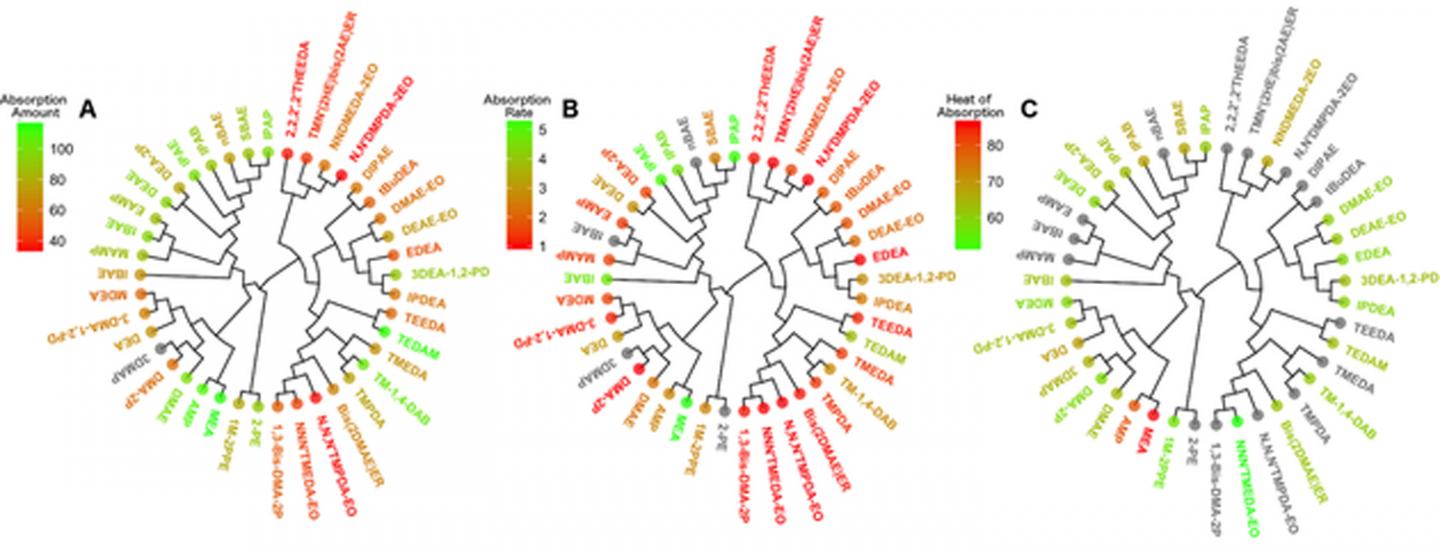
A proof-of-concept molecular modeling study from North Carolina State University analyzes the efficiency of amine solutions in capturing carbon dioxide. This series of new supercomputer models is the first step toward the design of cheaper, more efficient amine chemicals for capturing carbon dioxide - and reducing harmful CO2 emissions - in industrial installations.
Industrial scrubbers use chemical solutions to capture carbon dioxide (CO2) from fuel and combustion gas. The scrubbers are a commonly used method for decreasing carbon emissions from industries such as coal-fired power plants, which produce more than 14 billion metric tons of carbon each year. However, amine scrubbing is a costly process, so researchers are constantly looking for new amine chemicals with more desirable qualities such as fast absorption rates, high CO2 capacity, and low heat of reaction.
Denis Fourches, assistant professor of chemistry at NC State, and postdoctoral researcher Melaine Kuenemann wanted to find out if they could create supercomputer models that could predict an amine's absorption properties based on its chemical structure.
First, the researchers compiled information on 41 publicly available amine solutions and all their chemical and absorption properties. They analyzed the chemical and structural characteristics of each amine, and grouped them into families of chemicals with similar structural properties. Then they looked at how well and how quickly these amines could absorb carbon. Using this data, they created a series of models - known as a quantitative structure-property relationships, or QSPR models - that can predict the amines' CO2 absorption properties solely based on amines' structural characteristics.
These models utilize machine-learning techniques - the same ones used by companies like Netflix or Amazon that "learn" a customer's preferences and make recommendations based upon that data - in order to predict which chemical structures are likely to have the best overall CO2 absorption properties. The models were found to be capable of reliably discriminating between amines with high absorption properties versus those that were less efficient.
"This work is the first attempt to develop computer models for fully evaluating and predicting carbon dioxide absorption properties of amine solutions," Fourches says. "The next step for us is to utilize these [super]computer models to screen a virtual library of hundreds of thousands of new amines, and identify some new amine candidates predicted to have way better carbon absorption properties.
"If you had to test all of these thousands of compounds experimentally, it would take decades of work," Fourches continues. "With the powerful [super]computers we have access to, this virtual screening can be done in a matter of days and is very inexpensive. This is a game changer for designing and prioritizing new compounds."
The research appears in Molecular Informatics. The work was funded by the NC State Chancellor's Faculty Excellence Program.

